Abstract
Background: For patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who are eligible for autologous stem cell transplant (ASCT), salvage immunochemotherapy, such as rituximab, dexamethasone, cytarabine, and oxaliplatin or carboplatin (R-DHAX/C), followed by consolidation with high-dose therapy (HDT) and ASCT, is part of the standard of care. However, this treatment plan is unsuccessful in more than half of patients. Novel treatment options are needed to achieve improved responses and more favorable long-term outcomes. Epcoritamab, a subcutaneously administered bispecific antibody that targets CD3 and CD20, has demonstrated clinically meaningful antitumor activity both as a single agent (in the first-in-human phase 1/2 EPCORE NHL-1 trial; NCT03625037) and in combination with standard of care therapies (in the phase 1/2 EPCORE NHL-2 trial; NCT04663347) across B-cell non-Hodgkin lymphoma subtypes. Here we present updated results from arm 4 (epcoritamab + R-DHAX/C) of the EPCORE NHL-2 trial, which is the first clinical study of a bispecific antibody in combination with salvage immunochemotherapy in this high-risk, refractory, ASCT-eligible population.
Methods: Adults with R/R CD20+ DLBCL who were eligible for HDT-ASCT were treated with standard R-DHAX/C and subcutaneous epcoritamab (21-d cycles: QW, cycles 1-3). If HDT-ASCT was deferred, patients could continue epcoritamab monotherapy (21-d cycle: QW, cycle 4; 28-d cycles: Q2W, cycles 5-9, and Q4W, cycles ≥10) until disease progression or unacceptable toxicity. Corticosteroid premedication and step-up epcoritamab dosing were required in cycle 1 to mitigate CRS. Response was determined by PET-CT using Lugano 2014 criteria.
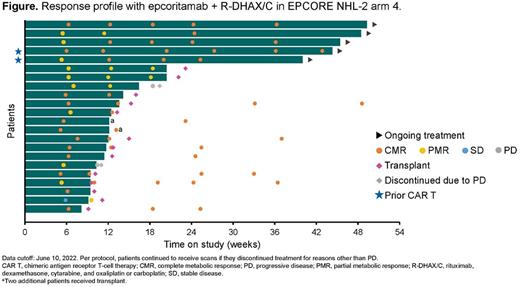
Results: As of the data cutoff on June 10, 2022, 29 patients had received epcoritamab + R-DHAX/C. The median age was 58 y (range, 28-75), 72% of patients had received 1 prior line of therapy, 28% had received 2 or 3 prior therapies, 34% had transformed disease, 66% had primary refractory disease, and 59% were refractory to their last line of therapy. Three patients had prior CAR T-cell therapy. The median duration of follow-up was 9.2 mo (range, 1.7-14.2). The most common treatment-emergent AEs (TEAEs) of any grade were thrombocytopenia (69%), anemia (45%), CRS (41%), neutropenia (41%), nausea (34%), and fatigue (28%). All CRS events were low grade (grade 1 in 31% of patients, grade 2 in 10% of patients) and resolved, with a median time to resolution of 2 d (range, 1-8). Most CRS events occurred after the first full dose. One patient experienced a grade 2 ICANS event, which resolved but led to treatment discontinuation. No clinical tumor lysis syndrome was observed, and no fatal TEAEs were reported. Of the 26 response-evaluable patients (Figure), 15 (58%) proceeded to transplant per protocol; for these 15 patients, the overall response rate (ORR) with the combination of epcoritamab + R-DHAX/C was 100% (15/15), with 80% (12/15) achieving a complete metabolic response (CMR) and 20% (3/15) achieving a partial metabolic response (PMR). Patients continuing epcoritamab monotherapy instead of HDT-ASCT had an ORR of 64% (7/11), with 45% (5/11) achieving a CMR and 18% (2/11) achieving a PMR. The primary reason for a patient not proceeding to HDT-ASCT was patient choice. Median duration of response was not reached among the 7 responders continuing epcoritamab monotherapy. In the response-evaluable population overall, the ORR was 85% (22/26); rates of CMR and PMR were 65% (17/26) and 19% (5/26), respectively.
Conclusions: Subcutaneous epcoritamab in combination with R-DHAX/C demonstrated a manageable safety profile in ASCT-eligible patients with R/R DLBCL. Importantly, no new safety findings were observed. ICANS was rare, and all CRS events were low grade and resolved. High ORRs and CMR rates were observed. As of the data cutoff date, most patients had proceeded to ASCT or remained on epcoritamab in CMR. In conclusion, epcoritamab is well suited for salvage combination therapy.
Disclosures
Abrisqueta:AbbVie: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Sandoz: Honoraria; Incyte: Consultancy; Janssen: Consultancy, Honoraria, Speakers Bureau. Cordoba:Pfizer: Research Funding; Kite: Consultancy, Speakers Bureau; Takeda: Consultancy; GenMab: Consultancy; Incyte: Consultancy; Janssen: Consultancy, Honoraria, Speakers Bureau; BeiGene: Consultancy; Lilly: Consultancy; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Celgene: Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Gilead: Honoraria; AbbVie: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau. Falchi:Genmab: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Genetech: Consultancy, Research Funding; Roche: Consultancy, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees. de Vos:BeiGene: Other: Participation on a Data Safety Advisory Board. Nijland:Genmab: Consultancy; Takeda: Research Funding; Roche: Research Funding. Wu:Genmab: Current Employment. Bykhovski:Genmab: Current Employment. Wang:Genmab: Current Employment. Rana:Genmab: Current Employment. Phillips:Xencor: Consultancy; Curis: Consultancy; Celgene: Consultancy; Kite/Gilead: Consultancy; Genmab: Consultancy; TG Therapeutics: Consultancy; Pharmacyclics/Janssen: Honoraria; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Epizyme: Consultancy; Eli Lilly: Consultancy; Abbvie: Consultancy, Research Funding; ADCT: Consultancy; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Research Funding; Beigene: Consultancy; Bayer: Consultancy, Research Funding; Incyte: Consultancy; Lymphoma & Myeloma Connect: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal